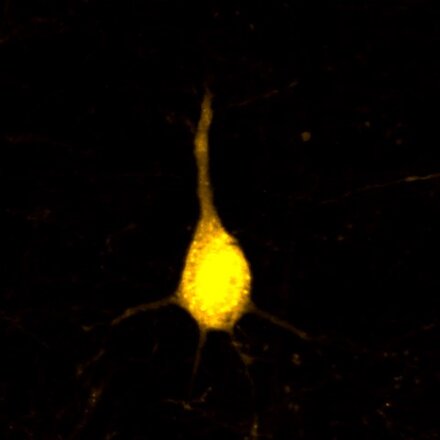
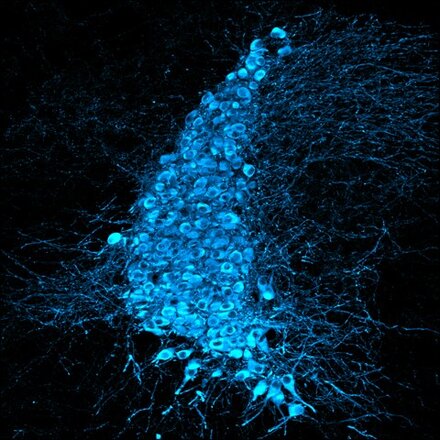
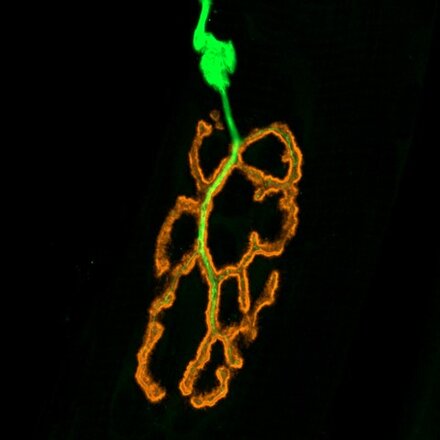
Presentation
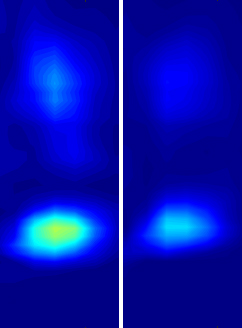
Comodulograms from a healthy model (left) and a model of ALS (right) showing decreased theta-gamma phase-amplitude coupling in the disease.
The term amyotrophic lateral sclerosis (ALS) derives from the initial histological description by French neurologist Jean-Martin Charcot in 1869, who reported two pathological features in patients' spinal cords: 1) the degeneration, in the lateral columns, of the corticospinal tract (lateral sclerosis, resulting from the degeneration of upstream corticospinal neurons), and 2) the disappearance, in the ventral horns, of spinal motor neurons (responsible for striated skeletal muscle denervation and amyotrophy, downstream). This initial histological definition still corresponds today to the clinical definition of the disease, since ALS is diagnosed when signs of degeneration of corticospinal neurons (CSN, or upper motor neurons) and motor neurons (MN, or lower motor neurons) are seen in combination.
The duality of neuronal damage that characterizes ALS raises the question of the relative contribution of each neuronal type (CSN versus MN), and of the cerebral cortex versus the spinal cord and skeletal muscles. While preclinical research into ALS has long neglected the cerebral cortex, a growing body of evidence from recent clinical studies now suggests that the disease may originate in the cerebral cortex.
In this context, the "Cerebral Cortex & ALS" axis directly investigates the contribution of the cerebral cortex to the onset and progression of the disease, using complementary technical approaches such as genetics, transcriptomics, molecular biology and electrophysiology, employed both on ALS patients and volunteer controls, as well as on preclinical models of the disease.
A better understanding of the origins and mechanisms of propagation of ALS will ultimately enable us to discover and test new diagnostic and prognostic biomarkers for the disease, and to design, test and propose new therapeutic approaches for patients suffering from familial or sporadic forms of ALS.